A/Prof. Ken Rodgers School of Life Sciences

Learning Objectives
- Understand the effect of pH partitioning and lipid solubility on drug absorption
- Describe factors affecting the absorption of drugs via oral administration especially first-pass metabolism
- Understand the suitability, limitations and precautions of various routes of administration
- Outline the concept of bioavailability
References
- Rang HP, Dale MM, Ritter JM, Flower R and Henderson G (2015) Pharmacology, 8th Edition, Churchill Livingstone, Sydney.
- Absorption and distribution of drugs– Chapter 8
Drug Absorption
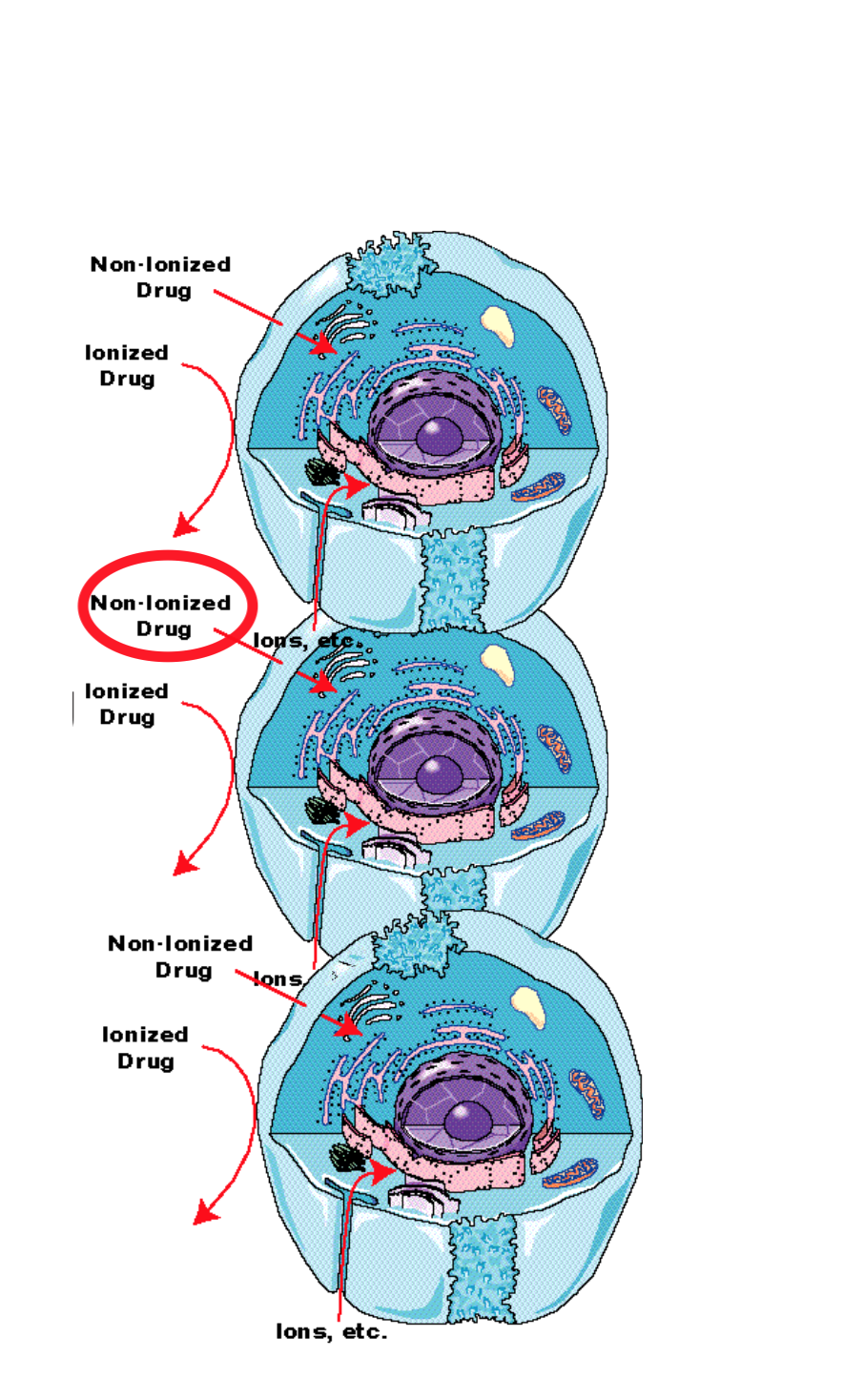
Absorbtion occurs mainly by diffusion through membranes
- If a drug has a low lipid solubility it will be poorly absorbed from gut (eg tubocurarine)
- Exception: Very small molecules may be able to penetrate poses in the mebrane (rare)
- Exception: If a drug is similar to a natural molecule that is transported on a carrier it could be absorbed by carrier-mediated (pump) transfer (eg levodopa, fluorouracil)
Passage of Drugs Across Membranes
- Cell membranes form barriers between aqueous compartments in the body
- Cell membrane are relatively impermeable to ionised drugs
- Special carriers and endo/exocytosis are probably not very important in drug absorption (few exceptions)
- Main process is probably pH partitioning
pH Partitioning

pH Partitioning
- Most drugs, or their salts, are weak acids/bases
- Thus the proportion of ionised to non-ionised drug depends upon the pH
- Ionised drugs are not very lipid soluble – only non-ionised form of drug crosses membrane readily

pH Partitioning
- Biological fluids (blood, stomach and intestinal contents, urine) have different pH values
- The pH of the solution will change the amount of drug that is ionised and will affect how and where drug is absorbed, distributed and how well it is excreted
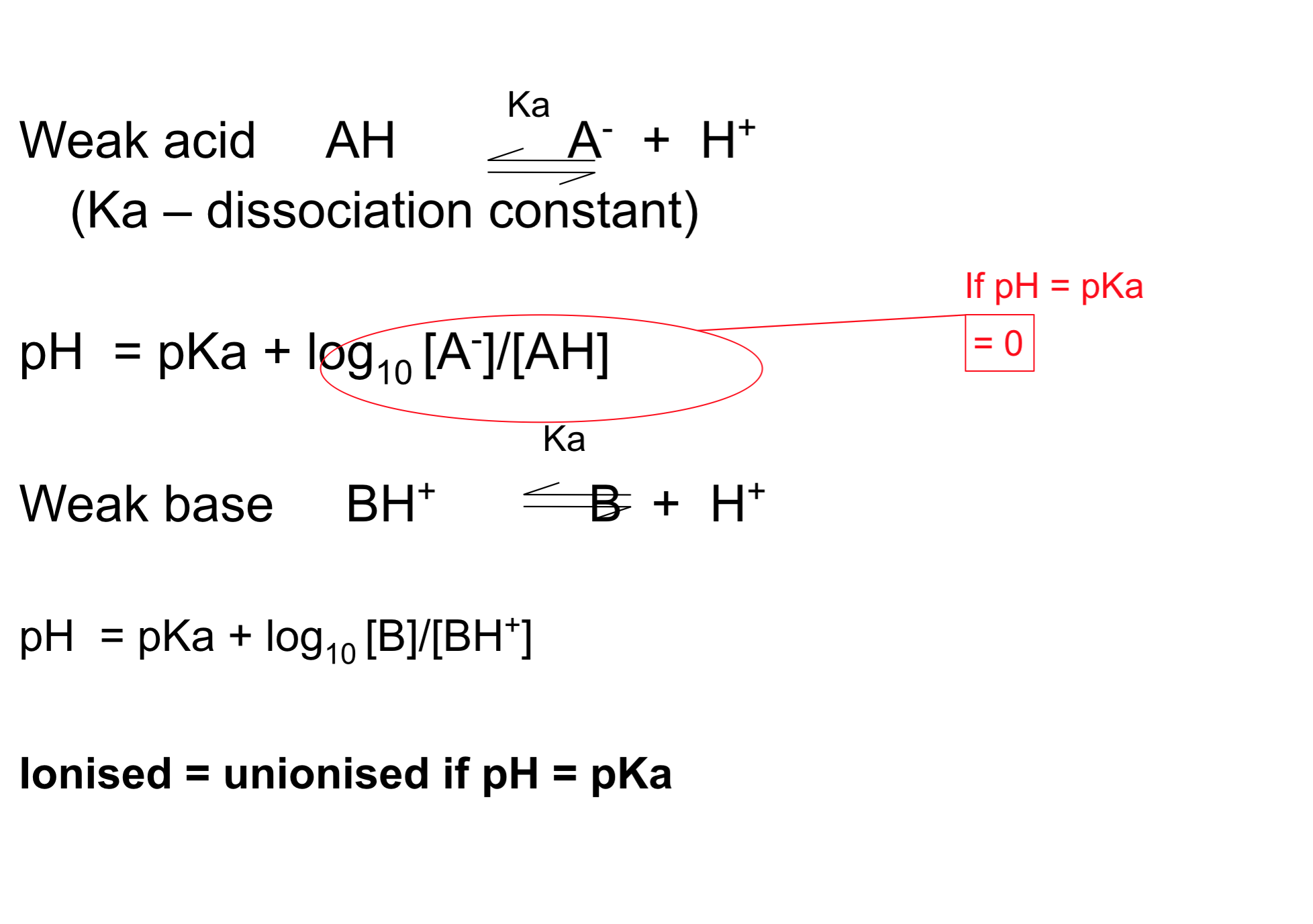
- For weak acids and weak bases the % ionisation is determined by the Henderson-Hasselbalch equation
pH Partitioning

pH Partitioning
- The degree of ionization is determined by the pKa of drug and pH of the solution
- If the pH of the solution is the same as the pKa of the drug then 50% will be ionised and 50% will be non-ionised.
- For acids:
- pKa – pH = log ( [non-ionised] / [ionised] )
- If pH = pKa then the log part of the equation = 0
- We know that log 1 = 0 so [non-ionised] / [ionised] must equal 1 so they must be the same
- For bases:
- pKa – pH = log ( [ionised] / [non-ionised] )
pH partitioning 4
- Eg. aspirin (weak acid), pKa = 3.5

pH partitioning 5
- Eg. pethidine (weak base), pKa = 8.6
Theoretical partition of a weak acid (aspirin) and a weak base (pethidine) between urine, plasma and gastric juice according to their pH differences. Numbers represent relative concentrations (total plasma concentration = 100). It is assumed that the uncharged species in each case can permeate membranes separating the compartments, and therefore reaches the same concentration in all three. Variations in the fractional ionisation as a function of pH give rise to the large total concentration differences with respect to plasma.
pH Partitioning 6
Applications of pH Partitioning
Applications of pH Partitioning 1
- Alkalinisation of urine (NaHCO3): increases rate of excretion of weak acids (more ionised) eg phenobarbital
Applications of pH Partitioning 2
- Acidification of urine (NH4Cl): increases rate of excretion of weak bases (more ionised)

Applications of pH Partitioning 3
- Increasing plasma pH (NaHCO3): will shift weakly acidic drugs from the CNS to plasma (more ionised)

Summary: cellular barriers
- To traverse cellular barriers (e.g. gastrointestinal mucosa, renal tubule, blood-brain barrier, placenta), drugs have to cross lipid membranes.
- Drugs cross lipid membranes mainly by (a) passive diffusion
- The main factor that determines the rate of passive diffusion across membranes is a drug’s lipid solubility (pH partitioning). Molecular weight is less important.

Summary: pH Partitioning
- Many drugs are weak acids or weak bases; their state of ionisation varies with pH according to the Henderson-Hasselbalch equation.
- Only the uncharged species (the protonated form for a weak acid, the unprotonated form for a weak base) can diffuse across lipid membranes; this gives rise to pH partition.
- Weak acids tend to accumulate in compartments of relatively high pH (highly ionised)
- Weak bases tend to accumulate in compartments of relatively low pH (highly ionised).
- When the pH = Pka the weak acid/base is 50% ionised
Oral Administration & Absorption

The oral route of drug administration
- Oral route (enteral), p.o. (per os)
- 1. most common route
- 2. usually safest
- 3. most convenient
- 4. most economical
- Surface area, not pH partition, is main determinant of site of absorption – villi/microvilli in small intestine > stomach
Rapid oral absorption
- So for rapid drug absorption, typically …
- Take tablet with a large glass of water (eg 200 mL)
- Take on an empty stomach eg at least half an hour before food (as long as gastric irritation is not a problem)
First-pass metabolism
- 1. First-pass metabolism
- Before entering the systemic circulation, blood leaving the GI tract passes through the liver
- Thus, drugs that are highly metabolised by the liver may attain very low circulating levels relative to those attained after parenteral administration
Difficulties with Oral Absorption
First-pass metabolism
- Difficulties with oral route 1
- 1. First-pass metabolism
- Before entering the systemic circulation, blood leaving the GI tract passes through the liver
- Thus, drugs that are highly metabolised by the liver may attain very low circulating levels relative to those attained after parenteral administration
Irregular absorption
- Difficulties with oral route 2
- 2. Irregular absorption depends on stomach contents
- delayed gastric emptying time
- altered stomach pH due to food
- decreased splanchnic blood flow in CHF
- complex formation of drug with food products (eg tetracyclines with milk)
Absorption of alcohol


Difficulties with oral route 3
3. Gastrointestinal irritation eg aspirin
4. Low pH may inactivate certain drugs eg penicillins, insulin
5. Particle size (small = more rapid absorption)
(eg see digoxin graph)
6. Requires patient compliance
Bioavailability

Oral bioavailability
- All the above factors influence oral bioavailability
- Fraction of orally administered drug that reaches the systemic circulation
- Two drugs with identical chemical composition that yield different blood concentrations and different effectiveness, differ in bioavailability (and are not bioequivalent)
- Varies between individuals
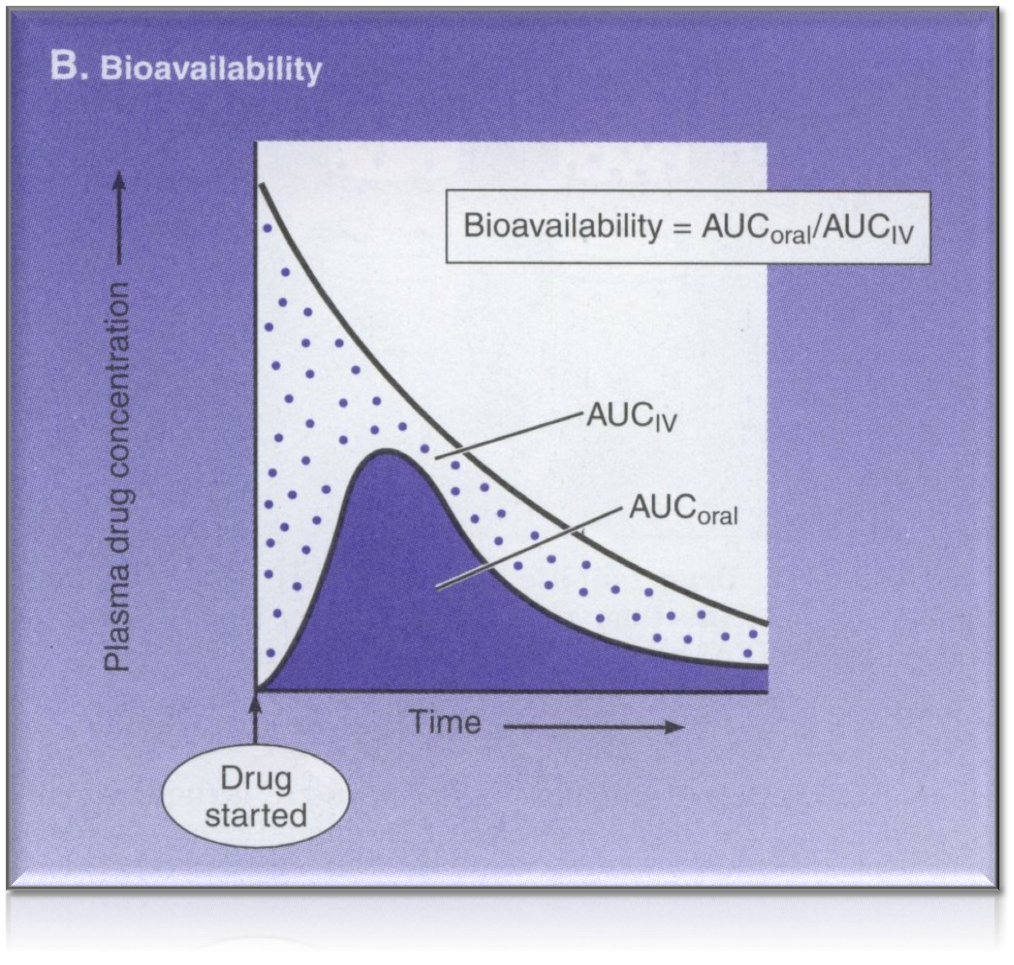
Overview of bioavailability
eg bioavailability of morphine via oral administration is only 20-33% when compared to IV administration
- Intravenous: 100% by definition
Intramuscular: 75 to <100%
Subcutaneous: 75 to <100%
Oral: 5 to <100%
Rectal: 30 to <100%
Inhalation: 5 to <100%
Transdermal: 80 to <100%
Other forms of drug administration

Sublingual (SL)
- Under the tongue
- Rapid absorption
- eg. glyceryl trinitrate
- Avoids exposure of drug to gastric pH
- Avoids first-pass metabolism
- Taste could be an issue
Intravenous
- Absorption pattern
- Precise, accurate and potentially immediate effects (absorption phase is bypassed)
- Suitable for large volumes and mixtures
- Special Utility
- Valuable for emergency use, permits titration of dose
- Usually required for high molecular weight protein and peptide drugs (eg. tPA)
- Limitations and precautions
- Greater risk of adverse effects
- High concentration attained rapidly
- Risk of embolism
- Must inject solutions slowly as a rule
- Not suitable for oily solutions or poorly soluble drugs
- Greater risk of adverse effects
IM and subcutaneous injection
- Absorption pattern
- Prompt absorption from aqueous solution, but slow and sustained from repository preparations
- Suitable for:
- Poorly soluble suspensions and slow release implants (sc)
- Moderate volumes and some irritating substances (im)
- Appropriate for self-administration (eg insulin)
- Limitations and precautions
- Not suitable for large volumes and pain and necrosis at injection sites for certain drugs (sc) eg thiopentone
- Precluded during anticoagulant therapy (im)
- May interfere with interpretation of certain diagnostic tests (eg. creatine kinase) (im)

Rectal (PR)
- Can be used for a local or systemic effect
- Unconscious patients, children with poor IV access, if patient is vomiting
- Easy to terminate exposure
- Absorption may be variable
- Good for drugs affecting the bowel such as laxatives / cathartics / drugs for ulcerative colitis

Spinal/Epidural
- Into spinal/epidural space for delivery of local anaesthetics/opioids for pain control
- Preferred over GA in lower abdominal or lower limb surgery or in child birth

Topical (TOP) 1
- Mucosal membranes
- Nasal, vaginal, etc.
- zolmitriptan spray – migraine
Topical (TOP) 2
- Skin
- 1. dermal (local)
- 2. transdermal (systemic)
- Stable blood levels
- No first pass metabolism
- Drug must be potent and lipophilic
- scopolamine – motion sickness
- oestradiol – hormone replacement
- fentanyl – pain
- clonidine – hypertension
- nicotine – tobacco withdrawal
- nitrates – angina