A/Prof. Ken Rodgers School of Life Sciences

Lecture Content
- To describe the structure and activity of receptors and ion channels
- To describe the various forms of receptor-effector linkages
- Receptor classification
- Other drug targets such as enzymes and transporters
References
- Rang HP, Dale MM, Ritter JM & Flower RJ, Henderson G (2016) Pharmacology, 8th Edition, Churchill Livingstone, Sydney
- Chapter 3. How drugs act: molecular aspects
Targets for drug action
Targets for drug action 1
Paul Ehrlich: ‘drug action must be explicable in terms of conventional interactions between drugs and tissues’
Targets for drug action 2
- Receptors
- 1. Ligand-gated ion channels (ionotropic receptors)
- 2. G-protein coupled receptors (metabotropic receptors)
- 3. Kinase-linked receptors
- 4. Nuclear receptors (intracellular receptors)
- Ion channels
- Voltage-gated ion channels
- Enzymes
- Transporters (carriers)
- Symports and antiports
Drug targets: receptors

Receptor Structure
- Receptors: An intracellular or cell-surface protein with which a drug or a signalling molecule interacts, to initiate a chain of biochemical events in the cell or organism
- Receptors are proteins, glycoproteins or lipoproteins – these proteins provide a complex 3D shape
- Four receptor SUPERFAMILIES are known
Ligand-Gated Ion Channels
Receptor Super Families

Ion channels
- Gateways in the cell membrane that allow the passage of particular ions
Ligand-gated: trigger is agonist binding
Voltage-gated: trigger is a change in transmembrane potential
Structure of ligand-gated ion channels


Ionotropic receptors
- Ligand-gated ion channel
- Location: membrane
- Effector: ion channel
- Coupling: direct
- Examples:
- Nicotinic acetylcholine (ACh)
- γ-Aminobutyric acid (GABA)
- Excitatory amino acids (eg. NMDA, aspartate)
- Glycine
- Timescale: extremely rapid cell activation with a time scale of milliseconds
- (note binding to receptor site is known as orthosteric binding as opposed to allosteric binding)
Ionotropic receptors
- Allows ions to cross membrane thereby causing:
- membrane polarisation (Cl–, K+)
- membrane depolarization (Ca2+, Na+)
- muscle contraction (nicotinic AChR of skeletal muscle)
- signal transduction (via Ca2+ mobilisation or influx)
Ionotropic receptors
- Example – Nicotinic AChR
- 288 kDa heteropentamer with 5 subunits (α, γ, α, β, δ, each 50 to 58 kDa) plus associated non-selective cation channel (for +ve charged ions)
- Each subunit contains 4 membrane spanning α-helixes to form pore ~0.7 nm in diameter
- 2 ACh molecules must bind to 2 α subunits for full activation conformational change to open ion channel (twisting of α subunits )
- Allows passage of Na+, K+, Ca2+ but at normal resting potentials, Na+ is major ion
Nicotinic ACh receptor

Nicotinic ACh receptor

Summary: ligand-gated ion channels
- sometimes called ionotropic receptors
- involved mainly in fast synaptic transmission
- several structural families exist, the commonest being heteromeric assemblies of four or five subunits, with transmembrane helices arranged around a central aqueous channel
- ligand binding and channel opening occur on a millisecond timescale.
- examples: nicotinic acetylcholine, GABA type A (GABAA) and 5-hydroxytryptamine type 3 (5-HT3) receptors.
Other ligand-gated ion channels
Receptor heterogeneity within families gives rise to subfamilies of different receptors in different tissues (eg nAChR in different regions of the brain

G-Protein coupled receptors
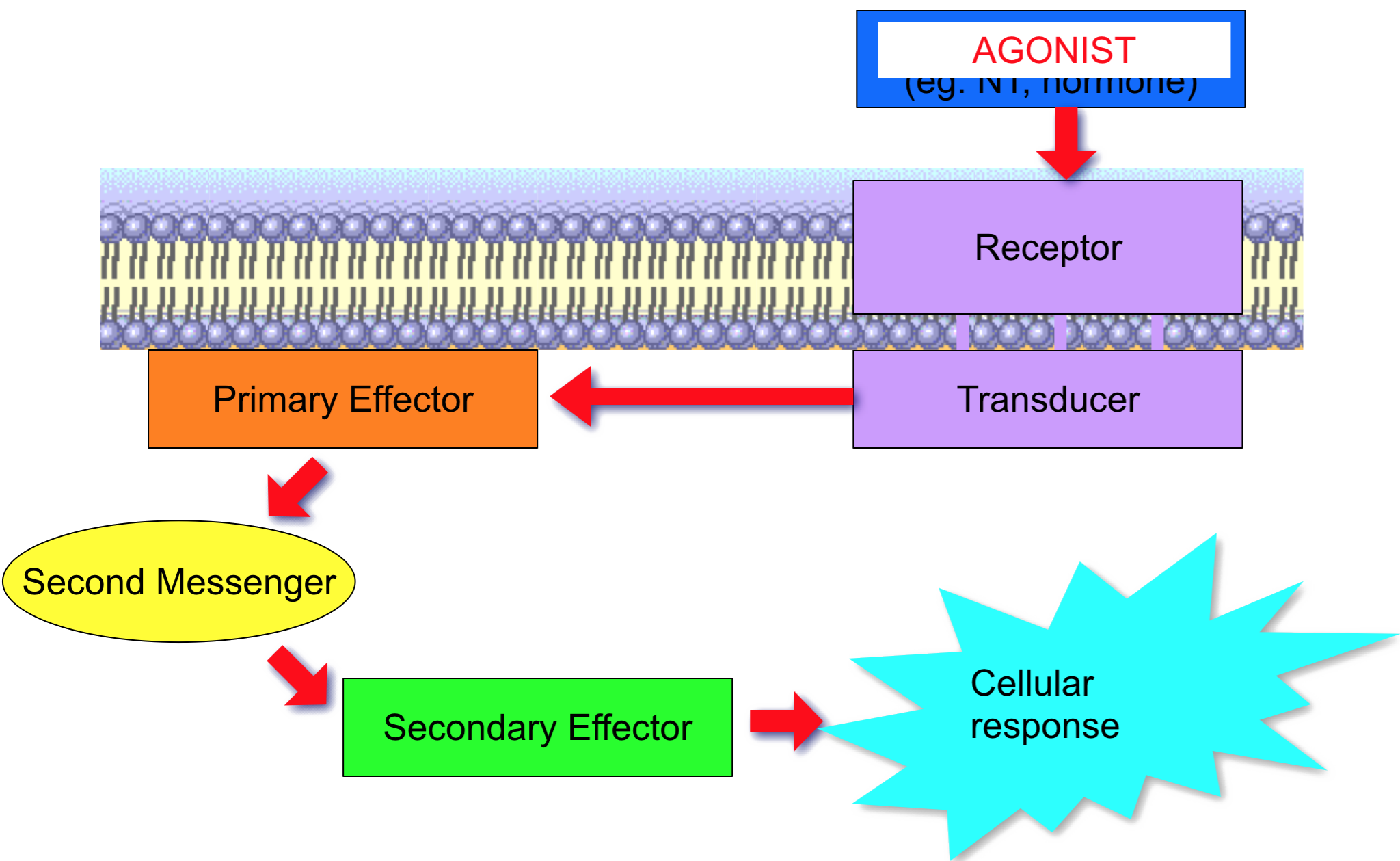
Types of receptor-effector linkage

The importance of G-proteins

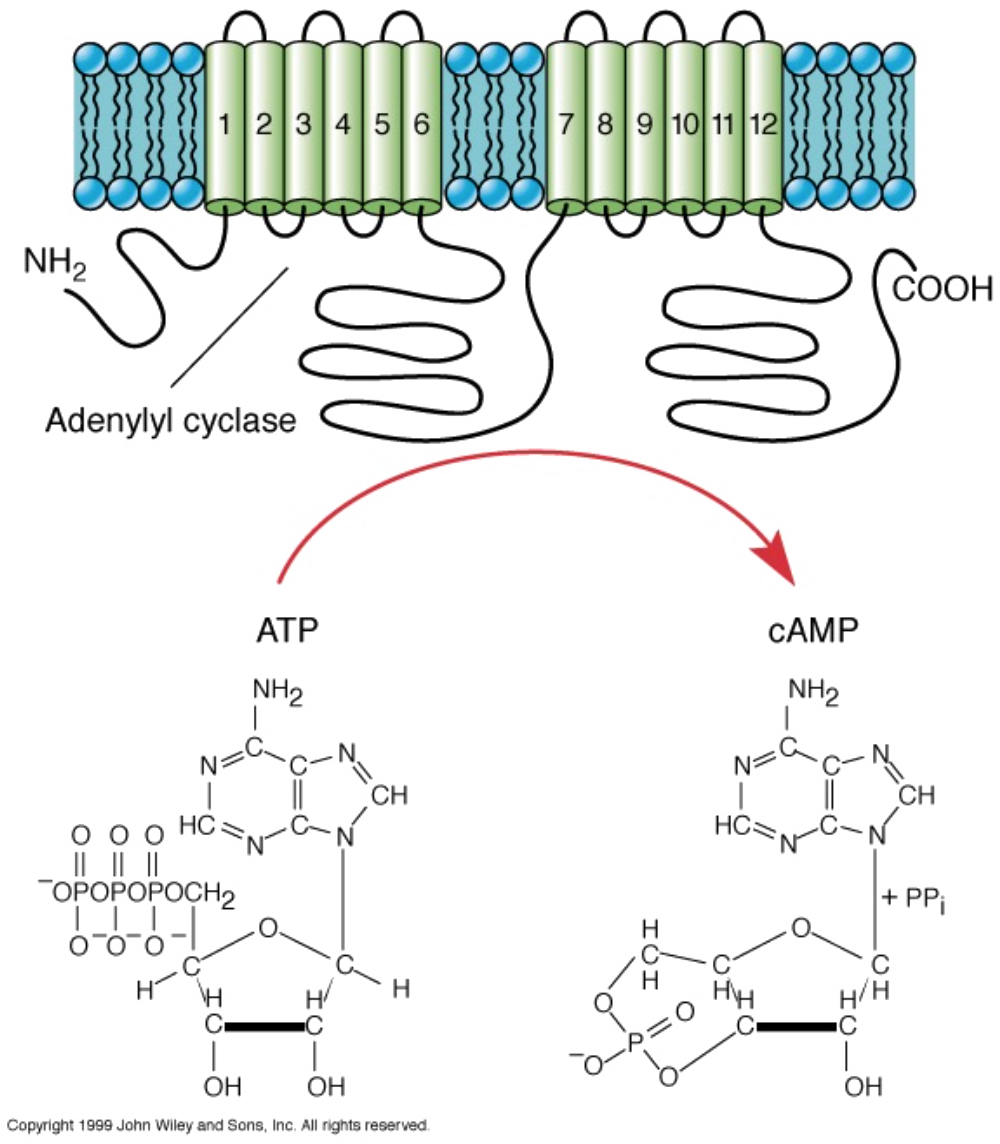
Structure of G protein coupled receptors
eg. muscarinic acetylcholine receptor


G-protein coupled receptors
- G–Protein Coupled Receptors (GPCRs) or ‘metabotropic’ receptors
- Location: membrane
- Effector: channel or enzyme
- Coupling: G-protein
(affinity for guanyl nucleotides (GDP/GTP)
- Examples:
- Muscarinic ACh
- Adrenoceptors
- Timescale: slow cell activation with a time scale of seconds

Structure of muscarinic AcH
- Seven transmembrane helix (heptahelical) structure
Family A: monoamine, neuropeptide and chemokine receptors
Family B: calcitonin and glucagon receptors
Family C: glutamate and GABA receptors
G-protein coupled receptors 2

G-protein coupled receptors 3
- Amplification of signal: one receptor can activate many G-proteins
- Active G-proteins can cause effector enzymes to produce many intracellular second messengers
- Principal second messengers:
- 1. Cyclic adenosine monophosphate (cAMP)
- 2. Ca2+
- 3. phosphoinositides (eg IP3 and DAG)
G-proteins: general scheme
G-protein coupled receptors 4
- G-proteins
- Involves intermediary G-proteins (Guanyl nucleotide-binding protein) present in receptor-membrane complex
- G-proteins serve 3 roles
- 1. G-proteins bind guanosine triphosphate (GTP) and GDP
G-proteins exist in 2 states:
- 1. G-proteins bind guanosine triphosphate (GTP) and GDP
- active form – GTP bound
- inactive form – GDP bound
- 2. G-proteins provide link between ligand-activated receptor and effector (enzyme/ion channel)
- 3. G-Proteins have intrinsic GTPase activity which spontaneously hydrolyses bound GTP to bound GDP (switch themselves off)
The function of G-proteins

G-proteins offer specificity
- Gi (‘i‘ for inhibitory) and Gs (‘s‘, for stimulatory) are heterotrimeric signal transduction complexes (αβγ)
- α-subunit interacts with a specific receptor (ie muscarinic, dopamine, noradrenaline) and a specific enzyme (eg. adenylate cyclase)
- On Gαi / Gαs activation, α subunit can dissociate from βγ

Primary effectors for GPCRs 1
- Targets for G-proteins
- Adenylate cyclase/cAMP system
- Phospholipase C/inositol phosphate system
- Regulation of ion channels
Calcium vs. cAMP
Kinase-linked receptors
Types of receptor-effector linkage

Structure of kinase-linked receptors


Kinase-linked receptors 1
- Location: membrane
- Effector: protein kinases
- Coupling: direct
- Examples:
- Insulin
- Growth factors eg. Epidermal growth factor (EGF), nerve growth factor, platelet derived growth factor (PDGF)
- Cytokine receptors eg. interferon-gamma (IFN-γ)

Kinase-linked receptors 2
- Timescale: cell activation with a time scale of minutes to hours
- These receptors consist of an extracellular hormone binding domain and a cytoplasmic enzyme domain
- Enzyme is usually a protein tyrosine kinase, but can be a protein serine kinase, a protein threonine kinase or guanyl cyclase (activation of receptor by phosphorylation)

Kinase-linked receptors 2
- Ligand binding
- Conformational change in receptor causes inactive monomeric receptor molecules to bind (noncovalently) to one another to form active dimer
- This brings together intracellular protein tyrosine kinase domains that become enzymatically active
- Tyrosine (Y) residues in cytoplasmic domains become phosphorylated (by each other)
- Enzymatic activity is activated to catalyse phosphorylation of substrate proteins
- Cross phosphorylation intensifies or prolongs allosteric action of hormone
Kinase-linked receptors 3

Insulin receptors

Insulin receptor
- Insulin receptor is an exception – it exists as a dimer
- Receptor is phosphorylated by cytosolic kinases
- Phosphatidylinositol 3-hydroxy kinase {PI(3)K}, makes PIP2,PIP3
- Grb2, Sos, activates Ras
- Activation of PI-PLC
Insulin receptor

Nuclear receptors
Types of receptor-effector linkage

Structure of nuclear receptors


Nuclear receptors 1
- Location: intracellular
- Effector: gene transcription
- Coupling: via DNA
- Examples:
- eg.Testosterone
- eg cortisol
- Mineralocorticoids
- eg aldosterone
- Hormones and vitamins
- eg. Vitamin D and thyroid hormone
- Sex steroids
- Glucocorticoids

Nuclear receptors 2
- Timescale: cell activation with a time scale of hours
- Agonist-receptor complex acting on DNA resulting in
- 1. transcription and translation of mediator proteins or
- 2. repression of expression of certain genes with inhibition of production of specific proteins
Nuclear receptors 3

Glucocorticoid receptors
Glucocorticoid receptor


Receptor classification
- Drugs are designed to bind to specific targets and these targets will only recognise certain drugs
- No drugs are completely specific – range of targets and actions at those targets (basis of adverse reactions)
- Drug receptors are receptors for endogenous mediators
- Identification and Classification can be:
- Based on effect of selective antagonists or representative agonists
- Differences in nucleotide sequence – functional differences? (molecular biology methodology)
- Orphan receptors exist
Drug targets

Drug targets: transporters
- A transport protein can transport molecules across membranes (this may be required if they are not very lipid soluble)
- Hydrolysis of ATP can provide the energy for transport of substances against their electrochemical gradient eg the sodium pump
- In some cases the transport of organic molecules is coupled to the transport of ions (usually Na+), either in the same direction (symport) or in the opposite direction (antiport)
Drug targets: transporters

Examples of drug targets

Lecture n* slides poll
Loading…