A/Prof. Ken Rodgers School of Life Sciences
Learning Objectives
- Recognise health care costs associated with Adverse Drug Reactions (ADRs)
- Outline the contribution of the following to the overall burden of preventable ADRs
- Age
- Pharmacogenetics
- Diseases
- Idiosyncratic reactions
- Drug interactions
- Identify mechanisms for specific clinically relevant drug interactions
- Identify issues of polypharmacy in the elderly
- Provide approaches to minimise ADRs
References
- Rang and Dales Pharmacology (8th ed)
- Chapters 11 and 57. Individual Variation and Drug Interactions. Harmful effects of drugs
- World Health Organisation
- The Food and Drug Administration (FDA) Center for Drug Evaluation and Research
- Therapeutic Goods Administration (TGA)
Intro to ADRs

An Adverse Drug Reaction (ADR) is:
– a response to a medication that is noxious and unintended
– occurs at doses normally used in human
Most ADRs occur in patients who are prescribed treatment within the limits of accepted medical clinical practice*
Definition from the World Health Organisation Available at: www.who-umc.org/defs.html
*Burgess et al., MJA, 182(6), 2005, 267-70

ADR – summary
- ADRs are among the leading causes of death in many countries
- In some countries ADR-related costs, such as hospitalization, surgery and lost productivity, exceed the cost of the medications
- A proportion of adverse drug reactions (ADR) are preventable
ADRs: Why are there so many?
- Two-thirds of patient visits to a doctor result in a prescription
- 2.8 billion outpatient prescriptions (10 per person in the United States) filled in 2000
- ADRs increase exponentially with 4 or more medications
- Important to reduce polypharmacy, but number of medications cannot always be reduced without doing harm
Reporting ADRs
The Therapeutic Goods Administration
- The Therapeutic Goods Administration (TGA) is a division of the Australian Government Department of Health and Ageing and is responsible for regulating therapeutic goods including medicines
- Any product for which therapeutic claims are made must either be listed or included in the Australian Registerof Therapeutic Goods before it can be supplied in Australia
- The TGA evaluates therapeutic goods before they are marketed and monitors products once on the market
- The TGA administers the Therapeutic Goods Act 1989

ADRs: Misconceptions about reporting
- All serious ADRs are documented by the time a drug is marketed – NO – the TGA monitor new drugs
- It is difficult to determine if a drug is responsible – NO – lots of data from lots of reports generate reliable data
- ADRs should only be reported if absolutely certain – NO (report on suspicion)
- One reported case can’t make a difference – NO – every report adds to the evidence
What to report?
YOU DO NOT NEED TO BE CERTAIN, JUST SUSPICIOUS!
- The TGA encourages the reporting of all SUSPECTED adverse reactions to new medicines, including vaccines, over-the-counter medicines, traditional or alternative remedies.
- Reports may be submitted:
- Using the ‘blue card from the TGA website
- Online on the TGA website
- By fax
- By email
What to report?
YOU DO NOT NEED TO BE CERTAIN, JUST SUSPICIOUS!

ADRs: Withdrawal from the Market
ADRs: Withdrawal from the market
- Drugs Removed/Restricted from U.S. Market due to Drug Interactions
- terfenadine (SELDANE®) February 1998
- mibefradil (POSICOR®) June 1998
- astemizole (HISMANAL®) July 1999
- grepafloxacin (RAXAR®) October 1999
- cisapride (PROPULSID®) January 2000
- FDA could not prevent co-prescription of these drugs with interacting drugs resulting in fatal interactions
terfenadine (antihistamine): increased risk of cardiotoxicity and arrhythmias listed interactions with around 70 drugs
ADRs: Withdrawal from the market
- Hailed as ‘super aspirin’ … ‘a truly effective weapon against the debilitating pain endured by arthritis’. Vioxx (a COX-2 inhibitor), used by 300,000 people in Australia and tens of millions worldwide, was pulled off the market in October 2004
- No one knows how many people died from strokes or heart attacks caused by Vioxx.
- ‘It would make 9/11 look like nothing’, says a leading cardiologist in the US.

ADRs: Safety profile of new drugs
- Efficacy and safety of new drugs are established in clinical trials
- Most drugs approved by FDA/TGA with ~2000 patient exposures
- For drugs with rare toxicity, > 100,000 patients must be exposed to generate a signal i.e. after drug is marketed
- Important safety issues may not be identified because.
- Some drugs have rare toxicity profiles (bromfenac severe hepatotoxicity 1 in 20,000 patients – now withdrawn)
- Lack of information in pregnant women, children, the elderly, and those with coexisting illness
- No comprehensive data concerning drug interactions
Individual Variation of Drugs
Individual Variation to Drugs
- Inter- and intra-individual variation to drugs often substantial, leading to:
- Lack of efficacy (sub-therapeutic)
- Unexpected side-effects (adverse drug reactions – ADRs)
- Types of variability classified as:
- Pharmacokinetic (differences in concentration at site of action – ADME)
- Pharmacodynamic (different responses to same concentration)
- Pharmacokinetic and pharmacodynamic: effect is the same but might be less or more – longer or shorter ie. qualitatively the same.
- Idiosyncratic
- Qualitatively different

Individual Variation to Drugs
- Main causes of variability are:
- Age (neonatal vs. adult vs. elderly)
- Pharmacogenetics (genetic factors)
- Disease (eg. kidney or liver disease)
- Idiosyncratic reactions (rare fatal reactions)
- Drug interactions (eg. enzyme induction/inhibition, receptor antagonism)
Variability: Effects of Age
Effects of Age on Drug Action
- Many changes in drug action in elderly are due to degeneration of the function of heart (blood flow), liver (metabolism), kidney (filtration)
- Cardiac output declines with age with a decreased proportion of blood flow to kidney/liver
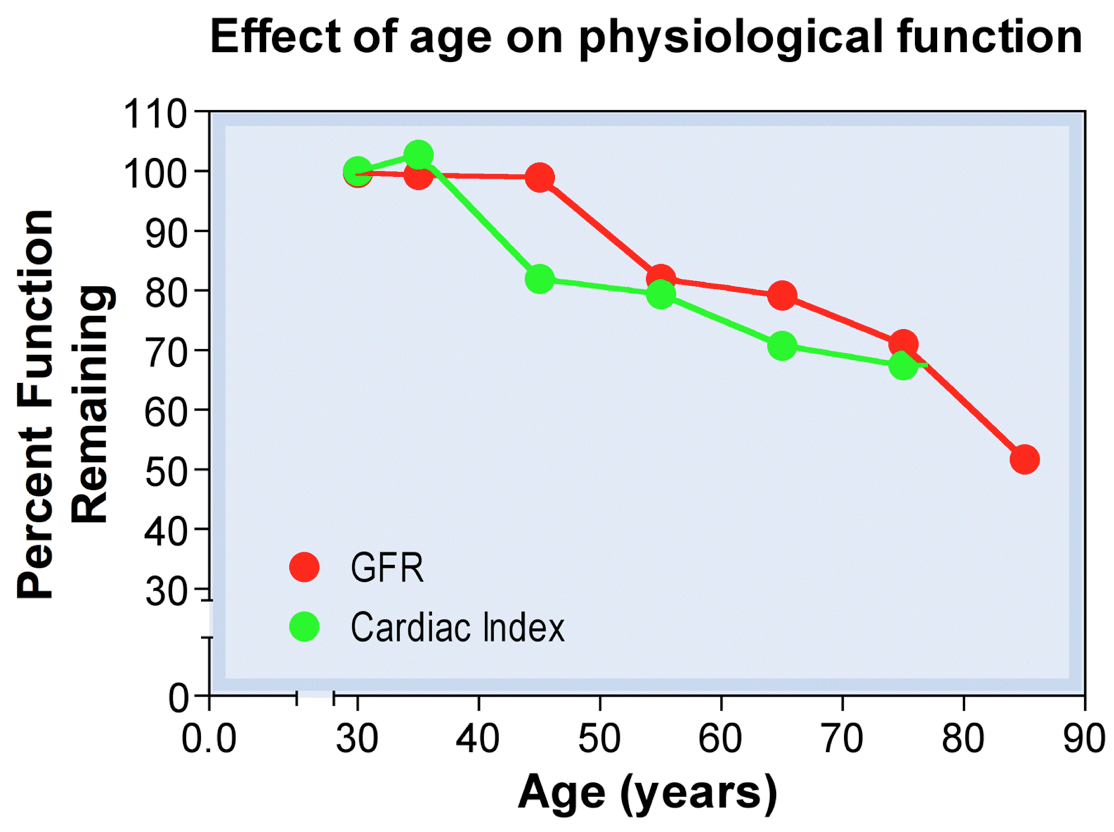
- GFR decreases with age with a reduced creatinine clearance rate
- Albumin concentration declines with age so less plasma protein binding and a greater concentration of free drug
- Variability in the function of above organs are more pronounced with disease-induced changes
Effects of Age: Renal Excretion
- Glomerular filtration rate (GFR) in the newborn is 20% of adults (100% after a week, 200% at 6 months)
- 100% at ~20 y.o.
- 75% at ~50 y.o.
- 50% at ~75 y.o.
- Decrease in GFR not reflected in plasma [creatinine] as creatinine synthesis declines with age
- Therefore clearance, not plasma levels, of creatinine must be checked
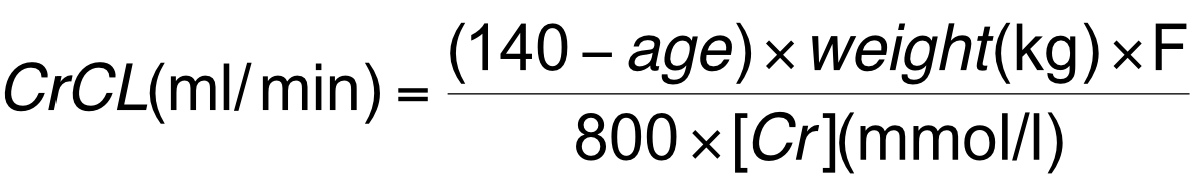
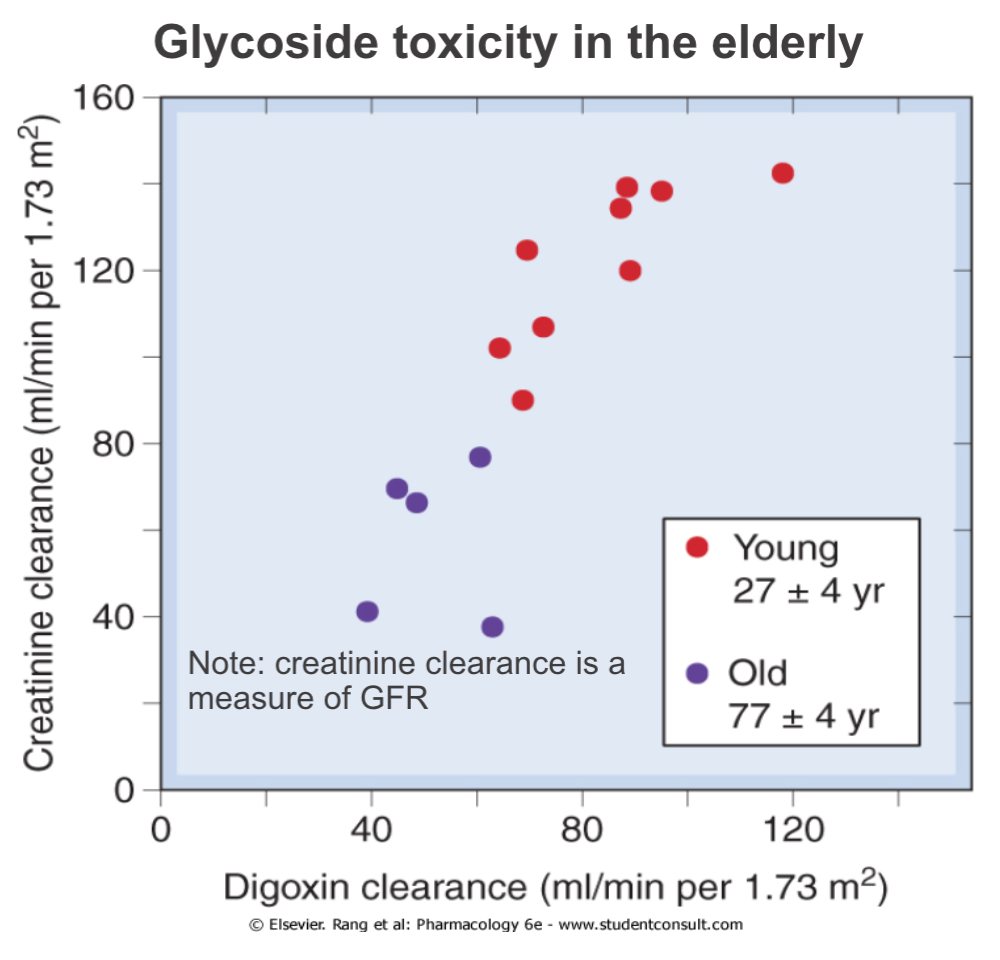
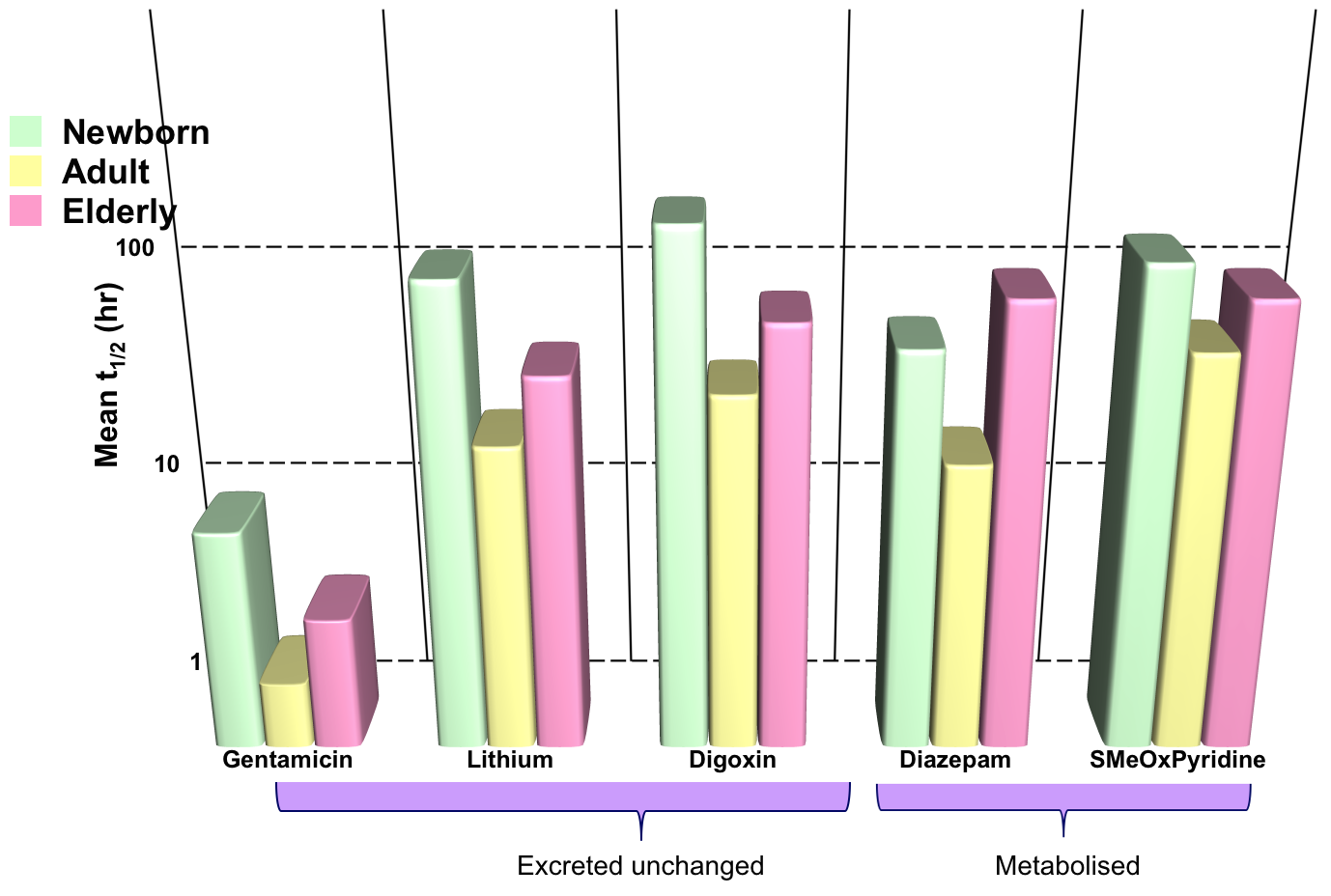
Effects of Age: Renal Excretion
- Drug elimination is less efficient in newborn babies and old people (elimination t1/2 longer)
- Results in greater and prolonged effects at the extremes of age
- Need to reduce and/or space out doses to avoid toxicity
Effects of Age: Drug Metabolism
- Enzymes have low activity in:
- neonates (>8 weeks to reach adult level)
- elderly (also with increasing variability)
- Includes: CYP450, glucuronyltrasferase, acetyltransferase, plasma ChE
Slow hepatic conjugation of:
- Chloramphenicol in babies – ‘grey baby’ syndrome
- Morphine during labour – respiratory depression in newborns
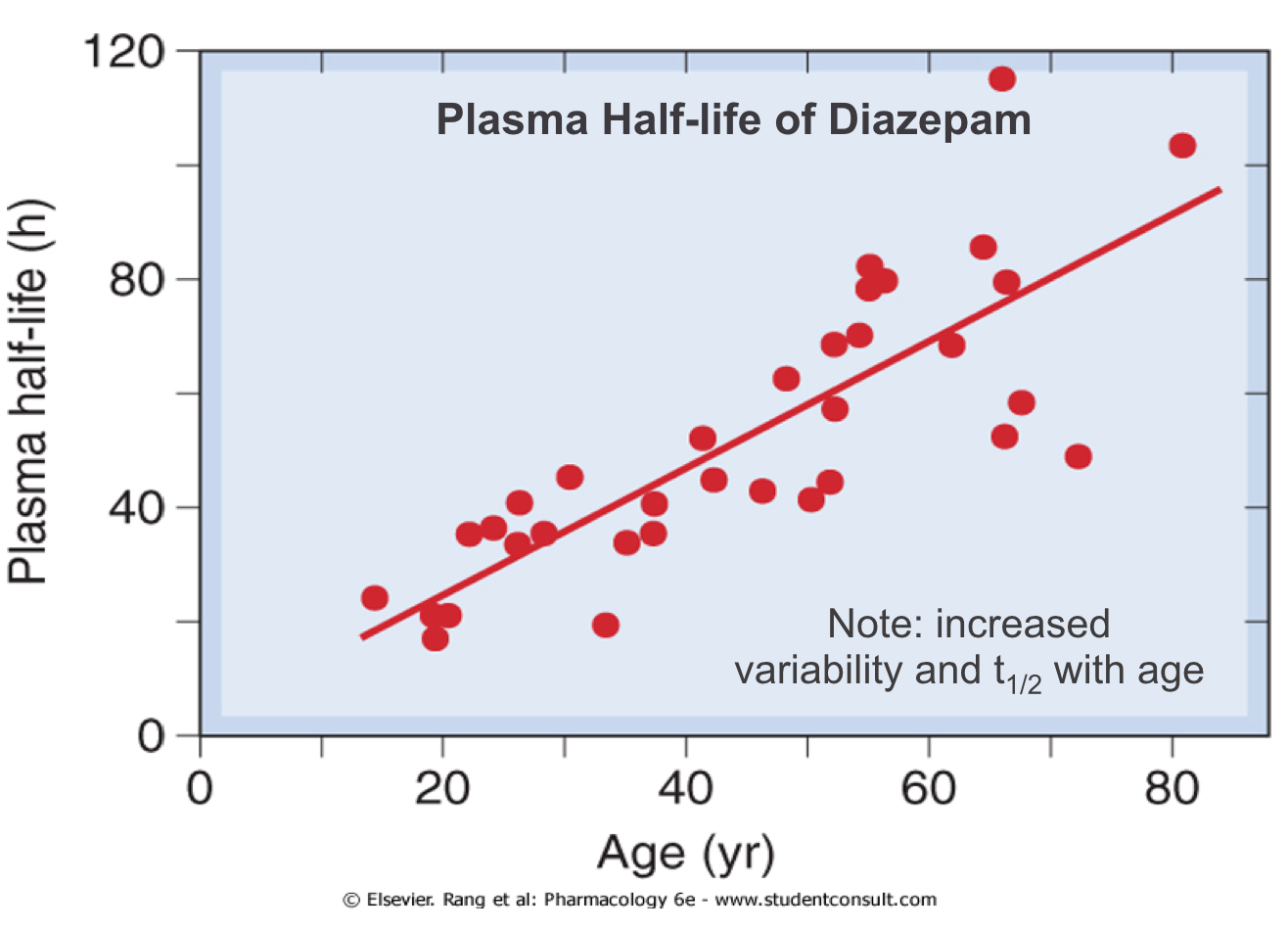
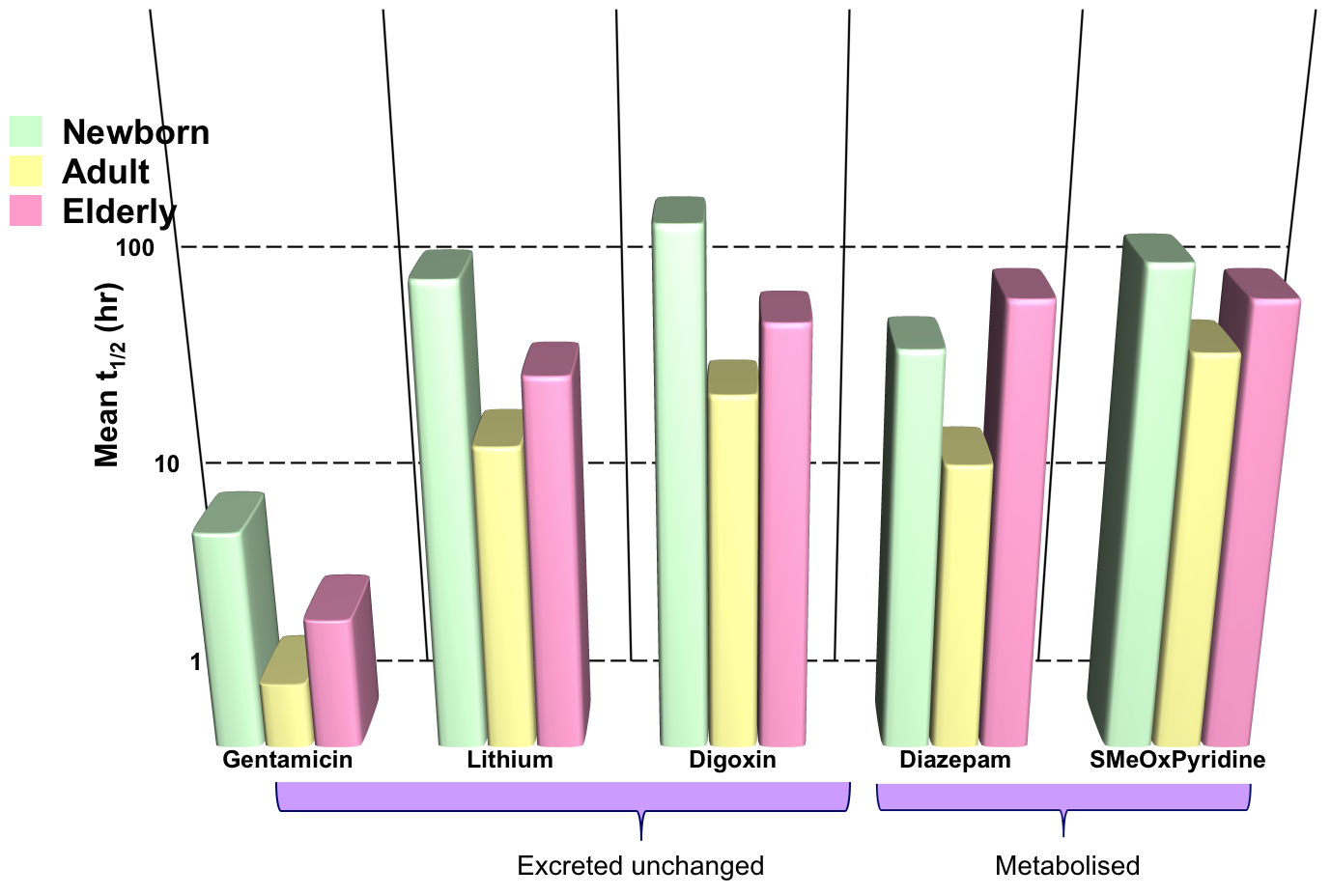
Effects of Age: Metabolism
- Drug elimination is less efficient in newborn babies and old people (elimination t1/2 longer)
- Results in greater and prolonged effects at the extremes of age
- Need to reduce and/or space out doses to avoid toxicity

Effects of Age: Drug Sensitivity
- The same plasma drug concentration can cause different effects in young and old for example:
- Benzodiazepines produce more confusion and less sedation in elderly than in young patients
- Hypotensive drugs cause postural hypotension more commonly in elderly than in young adults
Variability: Pharmacogenetics

Pharmacogenetics
- A large amount of individual variability to drugs is genetically determined
- Half-life of antipyrene (probe of hepatic drug oxidation) and warfarin (oral anticoagulant) are 6-22 times less variable in identical than in fraternal twins (same genes = same metabolism)
- Gene polymorphisms can affect an individuals susceptibility to ADRs
- Pharmacokinetic (eg. polymorphisms in genes encoding CYP450)
- Pharmacodynamic (eg. polymorphisms in drug targets such as receptors and enzymes)

Ethnicity
- Chinese subjects are considerably more sensitive to the cardiovascular effects of propranolol than white Europeans. Despite their increased sensitivity to this β-adrenoceptor antagonists (beta-blocker) , Chinese subjects metabolise propranolol faster than non-chinese people, implying that the difference relates to pharmacodynamic differences at or beyond the β adrenoceptors.

Personalised medicine
- Drug selection and dose are based on genetic information for each individual
- This could potentially remove the trial and error aspect of drug and dose selection.
- This approach is possibly over-hyped as in reality data are too difficult to interpret easily.
- Gene mutation could be advantageous for a population but have a negative influence on drug effect
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency confers partial resistance to malaria but increases susceptibility to heamolysis in response to drugs that cause oxidative stress. G6PD is also required to make on of the most important anti-oxidants in the body – glutathione.

Hla gene test
- Abacavir
- Is a reverse transcriptase inhibitor that is highly effective in treating HIV infection.
- Its use has been limited by severe rashes.
- Susceptibility to this adverse effect is closely linked to the human leukocyte antigen (HLA) variant HLAB*5701,
- Testing for this variant is used widely
- In this case testing will identify patients that will experience and adverse effect from the drug

HER2
- Trastuzumab
- Is a monoclonal antibody that antagonises epidermal growth factor (EGF) by binding to one of its receptors (human epidermal growth factor receptor 2 – HER2) which can occur in tumour tissue
- It is used in patients with breast cancer whose tumour tissue overexpresses this receptor. Other patients do not benefit from it.
- In this case testing will identify patients that will benefit from the drug.
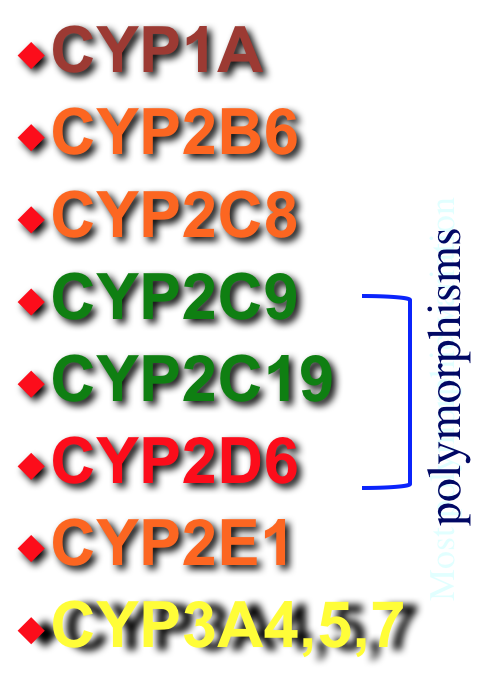
CYTOCHROME P450 ENZYMES
- Cytochrome P450 Nomenclature
- 12 families of enzymes are involved in metabolism in the liver of which 3 are involved in drug metabolism (CYP1, CYP2, CYP3)
- Each family has ~5 subfamilies (A, B, C, D, E)
- Individual isoenzymes are identified by a number (eg. CYP3A4, CYP2D6)
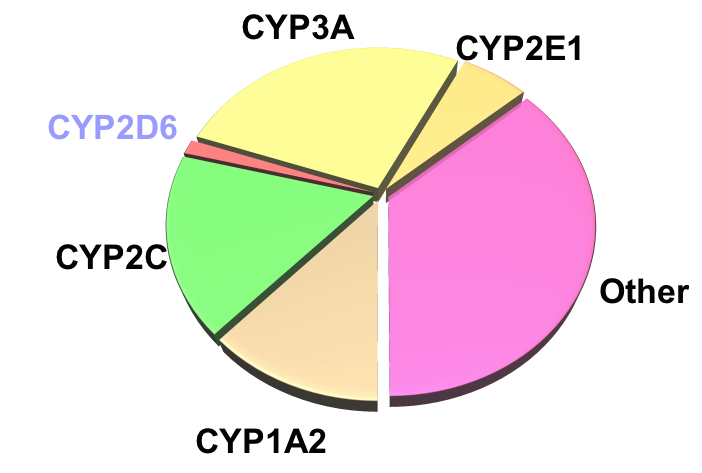
Drug Interactions: Metabolism
Relative Quantities of P450s in Liver:
Pharmacogenetics: Polymorphisms
- A trait that has differential expression in >1% of the population

Pharmacogenetics: Polymorphisms
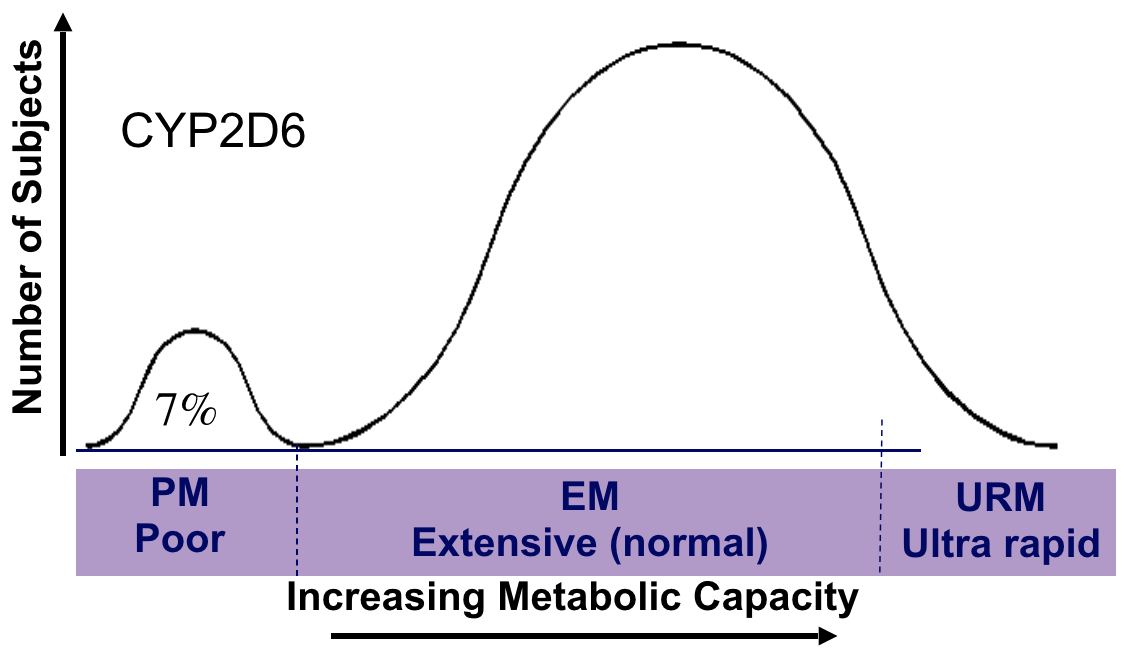
- CYP2D6 Extensive metaboliser (EM–normal)
- Codeine to morphine (rapid pain relief)
- Many drugs inactivated
- CYP2D6 Poor metaboliser (PM)
- Codeine to less morphine (less pain relief)
- Many drugs inactivated slowly (more toxicity)
- CYP2D6 PM 5-10% of Caucasians,1–3% Asians
- CYP2D6 URM in up to 30% of East Africans
Pharmacogenetics: Polymorphisms
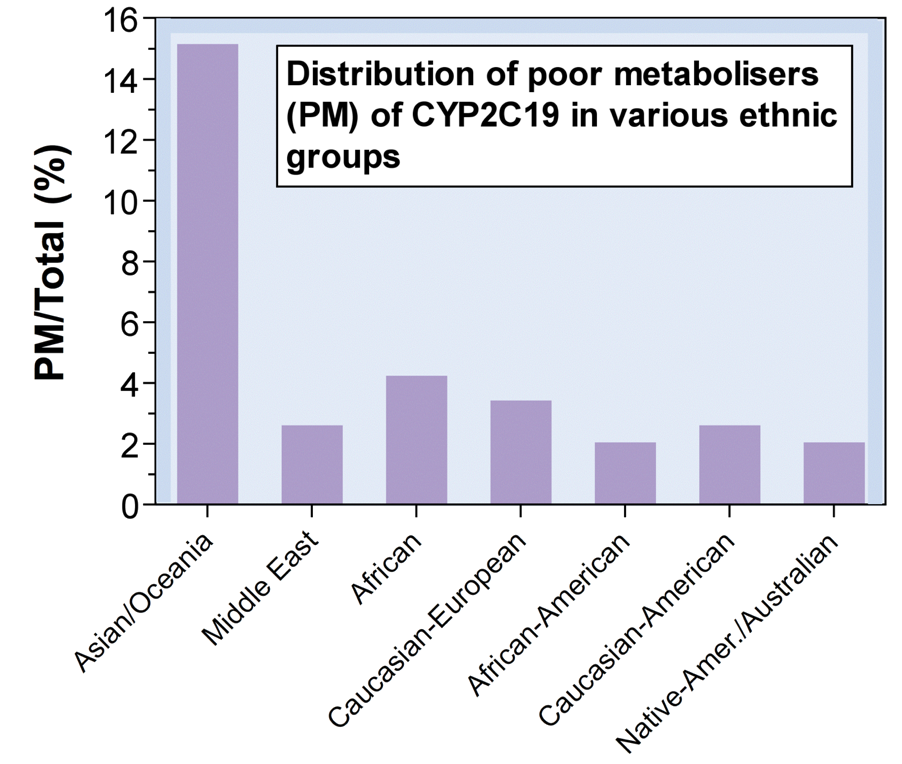
CYP2C19 PM in 15% of Asians, 3–5% Caucasians (Clin Pharmacokinet, 2002: 41(12): 913-958)
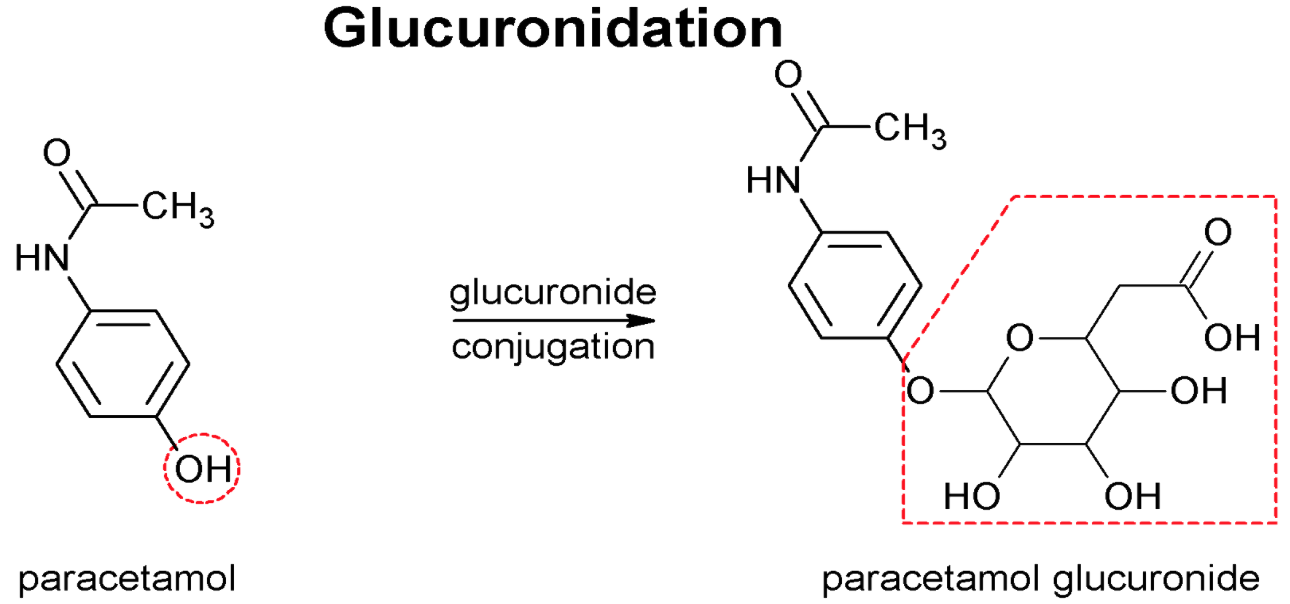
Phase 2 Reactions
Pharmacogenetics: Polymorphisms
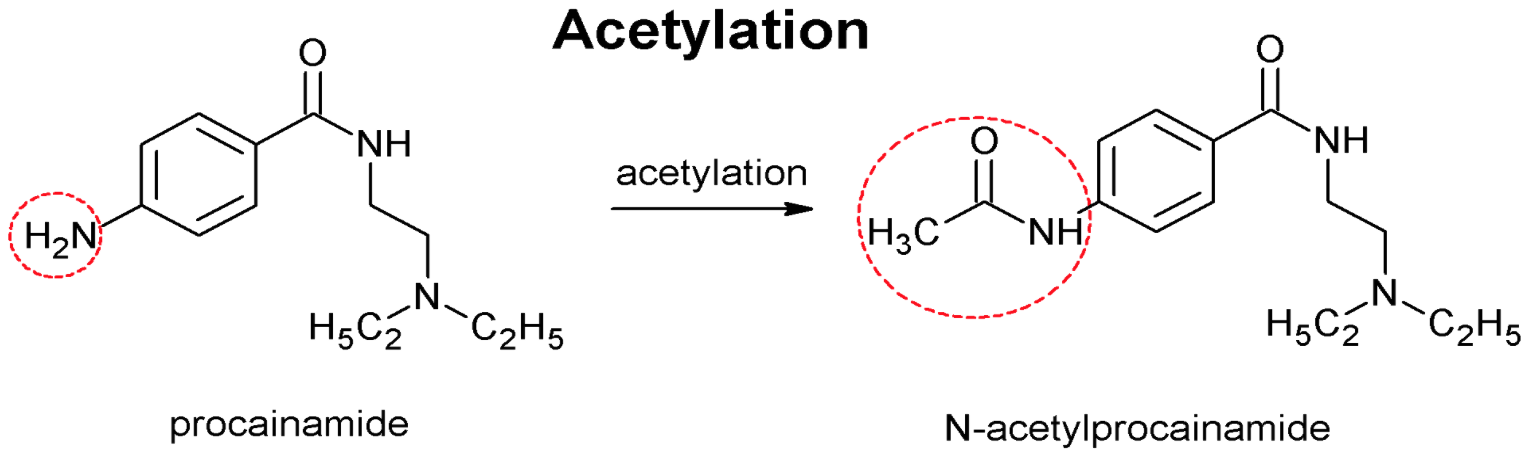
- Fast or slow drug acetylation is determined by single recessive gene associated with low hepatic acetyltransferase activity
- Acetyltransferase also important for metabolism of hydralazine, procainamide and various sulfonamides
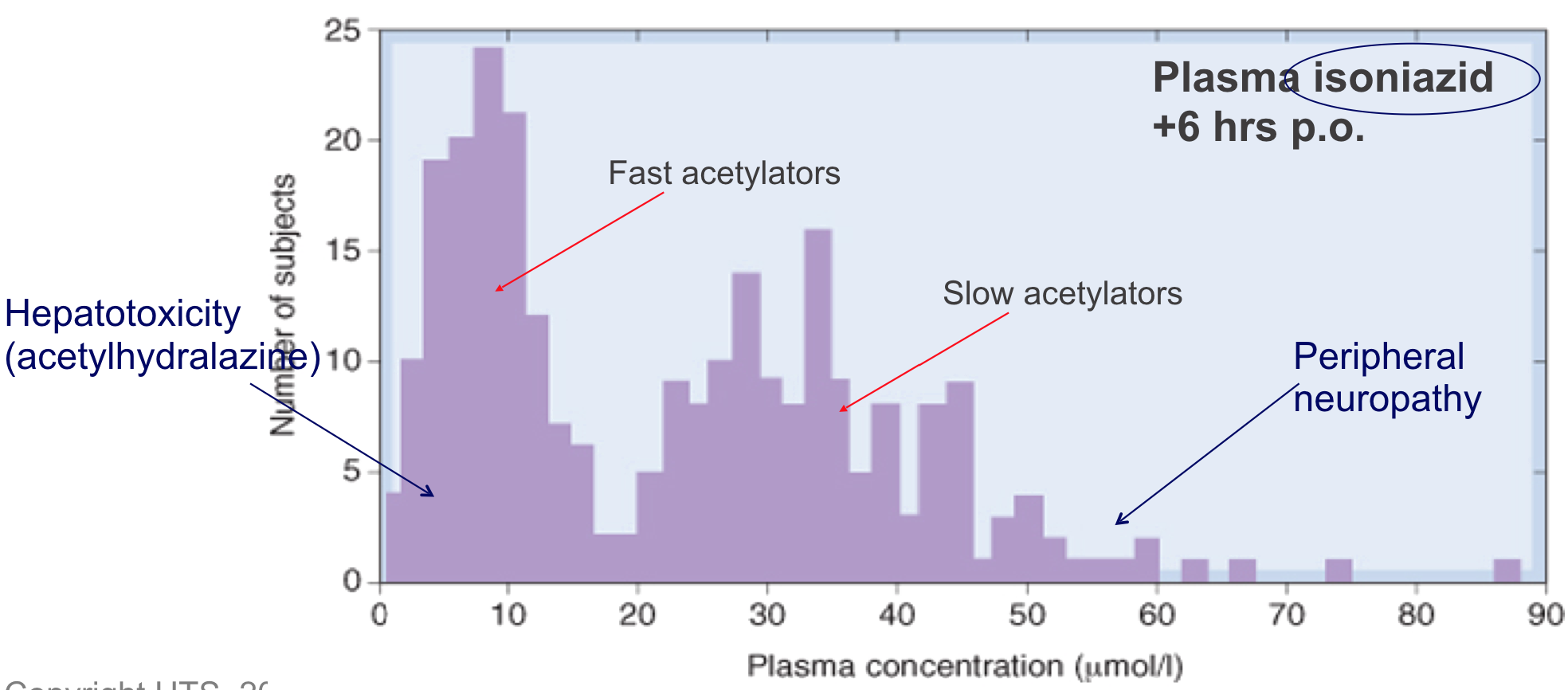

Pharmacogenetics: Polymorphisms
- 1 in 3,000 patients fail to hydrolyse suxamethonium rapidly – prolonged neuromuscular block (normally short-acting)
- Recessive gene for abnormal plasma cholinesterase
- Dibucaine inhibits the normal but not abnormal enzyme (70 to 90%)
- Dibucaine number = percentage inhibition of plasma ChE by 10-5 mol/l dibucaine
Suxamethonium is a short-acting neuromuscular blocker widely used for intubation in anaesthesia (wears off 10 min)
Variability: Disease and Idiosyncratic Reactions

Effects of Disease
- Liver and kidney disease
- Prolonged drug effects – toxicity
- Reduction in protein binding, liver/kidney blood flow, liver capacity
- Prolonged drug effects – toxicity
- Migraine and diabetic neuropathy
- Slowed drug absorption due to gastric stasis
- Heart failure
- Reduced liver perfusion – toxicity
- Mucosal oedema – reduced absorption
- Hyperthyroidism
- Increased sensitivity to pethidine (mechanism?)
- Hypothermia
- Reduced drug clearance
Idiosyncratic Reactions
- Harmful, sometimes fatal, reactions that occur in a small minority of individuals
- Qualitatively abnormal,
- Rare
- eg. Chloramphenicol induced aplastic anemia (bone marrow depression) in ~1 in 50,000 patients
- Reactions may occur with low doses
- Sometimes genetic factors may be responsible
- Primaquine, dapsone, doxorubicin, some sulfonamides and Vicia fava beans cause severe anemia in G6PD deficient Afro-Carribean men (deficiency in the antioxidant glutathione)
